Anterior Colporrhaphy
Authors
INTRODUCTION
To function normally, the vagina must have adequate length, elasticity, a well-estrogenated lining, and rest upon a responsive levator muscle plate. Symptoms of vaginal relaxation may include a sense of pelvic heaviness, recognition by the patient of a bulge or protrusion from the vagina, urinary incontinence, and dyspareunia. Surgically correctable complications of vaginal anatomy include relaxation or widening of the vaginal caliber, narrowing of the upper vagina, iatrogenic shortening of the vagina, stricture of the introitus, or complete absence of the vagina. Uncommonly, there may be duplications of the vaginal canal. A partition, or septum, may run the full length of the vagina and one side or the other may be obstructed. on occasion, a duplicated vagina obstructed near the introitus may be misdiagnosed as a cystocele. Despite minor improvements in vaginal support resulting from atrophy and narrowing of the vaginal introitus, once established a cystocele will not improve.
Surgical incisions of the anterior vaginal compartment are utilized for the following:
To repair a cystocele or enterocele
To correct urinary stress incontinence
To excise a suburethral cyst or diverticulum
To access the peritoneal cavity through the anterior cul-de-sac
To repair vesicovaginal fistulas
More commonly today, incisions of the anterior vaginal wall may be required to reduce the vaginal caliber for improved coital comfort. Incisions may be indicated for drainage of a hematoma in the retropubic space or wall of the vagina. The degree of severity of the cystocele must be evaluated with the patient at rest and during a forced increase in intra-abdominal pressure, as with a Valsalva maneuver. If a woman's symptom of a vaginal protrusion cannot be confirmed during examination in the lithotomy position she should be examined while standing, with her legs slightly apart. The size of the cystocele or vaginal protrusion may bear no relation to the sense of pelvic heaviness, or severity of urinary stress incontinence. Some women are oblivious to large protrusions from the vagina.
Before surgery the routine steps taken before any operative procedure should be carried out. The gynecologist should determine:
- The presence of any ureteral obstruction and hydroureter resulting from the passage of the ureter across the medial border of the levator muscle toward the sacculated portion of the bladder
- The presence or absence of any urinary or vaginal infection, decubitus ulcers, or suspicious areas of malignancy
- The exact nature of any urinary tract symptoms (e.g., incontinence, urgency, nocturia, dysuria)
- A reliable sexual history
The principal aim of any surgical repair of the anterior vaginal compartment includes restoration of adequate vaginal length and caliber, maintenance or restoration of urinary continence, and repair of the pelvic valvular mechanism. See elsewhere [] Informed consent should touch not only on possible complications, but as well on the patient's expectations. In many elderly women marked atrophy of the vaginal wall may accompany a cystocele. Presurgical treatment with estrogen should be carried out. The subject of this chapter concerns the correction of a cystocele, with or without accompanying urinary incontinence. (Note that a cystocele is often found in combination with uterine prolapse. See elsewhere in these volumes.)
TECHNIQUES OF ANTERIOR COLPORRHAPHY FOR CYSTOCELE REDUCTION
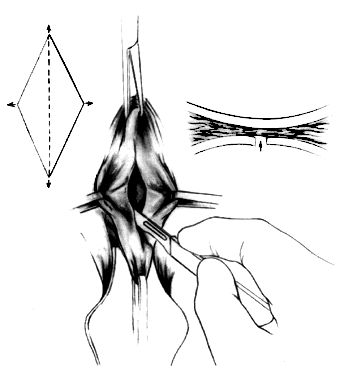
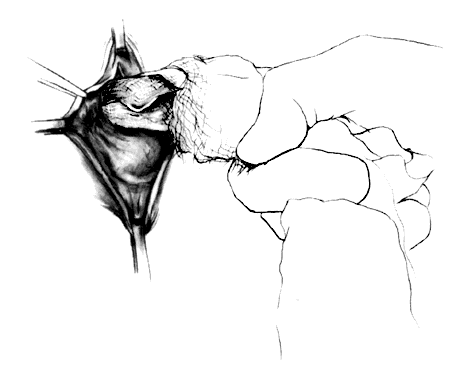
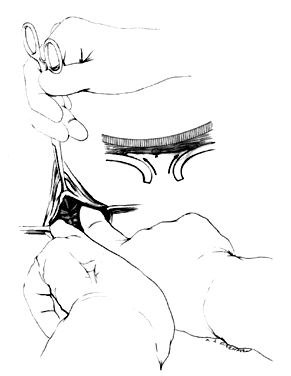
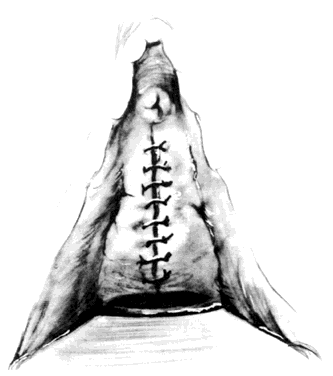
After a vaginal hysterectomy is completed, four Allis clamps are used to stretch the anterior vaginal wall into a diamond shape. With one stroke of a fresh scalpel, an incision is made through the full thickness and length of the anterior vaginal wall from 2-3 cm posterior to the external urethral meatus almost to the vaginal vault (Fig. 1). A scalpel is preferred because electrocautery can produce variable damage to tissues beyond the point of contact of the electrode. Tension on the four Allis clamps will cause the edges of the incision to separate readily. The bladder also can be separated from the vagina by tunneling between the two with closed scissors, a method slightly more tedious and less elegant than incision with a scalpel. The two lateral Allis clamps then are reapplied to the cut edges of the vaginal epithelium. The knife is drawn along the inner cut edge of the vaginal wall, loosening (with one or two strokes) the vesicovaginal connective tissues from the vaginal wall (Fig. 2). The surgeon's index finger, wrapped with a single layer of dry gauze, then is used to press and rotate against the vaginal flap, further separating these connective tissues (Fig. 3). As much of the vesicovaginal connective tissues as possible should remain attached to the base of the bladder. The separation of the connective tissues from the flaps of the vagina should proceed laterally only for approximately 1 inch on each side.
|
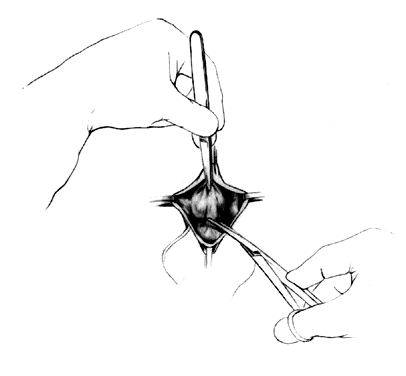
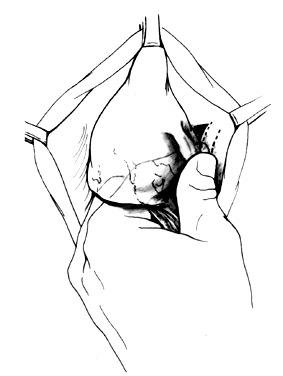
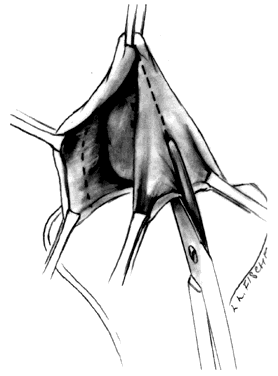
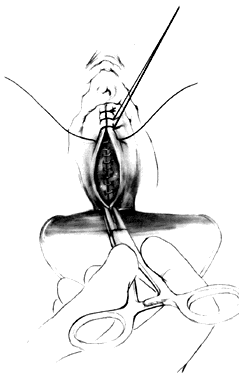
Attention is directed next to the area of the bladder remaining adherent in the midline at the vaginal vault. Midline traction upward on the bladder produces a line of tension between the vagina and vault, which is incised with a Mayo scissors pressing downward, creating a space in the midline bounded by the pillars of the bladder (Fig. 4). Allis clamps are reapplied on each side, and the bladder pillars are separated from the vaginal wall. To avoid injury to the bladder, the Mayo scissors are pressed firmly against the vaginal walls while the bladder is pulled to the contralateral side (Fig. 5). The ureters may be palpated at this point (Fig. 6). The first row of sutures is then placed through the vesicovaginal connective tissues. These are set approximately 1.5 cm apart, beginning at the urethrovesical junction. Success depends on the creation of a broad plate of vesicovaginal connective tissue beneath the bladder. Altering the direction of the suture path in the region of the urethrovesical junction and bladder pillars may give additional length to the plate of connective tissues underneath the bladder (Fig. 7).
|
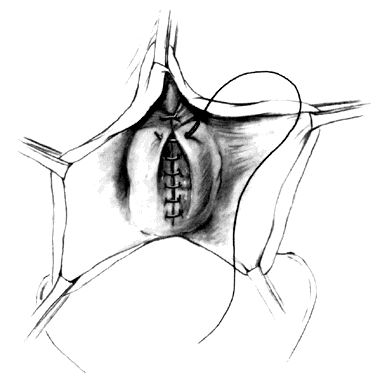
After completion of the first row of sutures, the surgeon's index fingers are used to press between the vaginal flaps and the connective tissue plate to provide access to the more lateral and denser layers of connective tissue (Fig. 8). The second and third layers of sutures further reduce the cystocele, buttress the repair, and promote hemostasis (Fig. 9). Additional dissection upward behind the pubic bone facilitates further retropubic urethral suspensions or the placement of paravaginal sutures. In most instances, sutures should not be placed directly into the wall of the urethra. Care must be taken not to straighten the urethrovesical junction, because such a maneuver can distort these tissues and result in urinary incontinence. Elliptical excisions of the anterior vaginal wall must be done carefully to avoid a persistent bulge or undesirable stenosis (Fig. 10).
|
At this stage, it is helpful to grasp each vaginal flap at the apex with an Allis clamp and to push them back in toward the lower sacrum (Fig. 11). With the vagina temporarily replaced to its normal position, one can estimate more accurately to what degree the vaginal flaps must be trimmed, because lengthening of the upper paracolpia may have caused a telescoping and redundancy of the vaginal cylinder, resembling a true cystocele. If the vaginal wall is not long enough to span comfortably between the pubic symphysis and posterior supports, attaching the apex of the vagina posteriorly pulls the urethra away from the symphysis, and may cause incontinence. Should this be recognized, additional length should be contributed to the anterior vaginal apex from the posterolateral fornices.2 Finally, persistent mobility of the vaginal vault after anterior colporrhaphy may indicate the need for additional fixation. Interrupted sutures are used to close the anterior wall, helping to preserve the length of the repaired connective tissues (Fig. 12).
Intra-abdominal pressure usually suffices to obliterate the space between the repaired vesicovaginal connective tissues and the vaginal wall without the need to suture these structures together. A vaginal pack is used only when an anterior repair is combined with a posterior repair, to keep the opposing suture lines from adhering to each other and obliterating the vaginal canal.
COMPLICATIONS
To guarantee an adequate repair, as much of the vesicovaginal connective tissues as possible should be left in contact with the bladder muscularis. On occasion, the thick bands of connective tissue exposed laterally may be lost, or perforated, as one dissects bluntly further laterally. Plication in several layers may compensate for some deficiencies in the thickness of the connective tissues. Injury to surrounding structures, such as the bladder, ureter, or urethra, diminishes as the surgeon gains experience. Shortening of the anterior plate of vesicovaginal connective tissues results in a shortened vagina, preventing efficient overlap between the anterior and posterior valve leaves. Shortening of the anterior vaginal wall also results in a more anterior cul-de-sac, increasing the likelihood of an enterocele. An adequate repair of the vagina depends on the formation of a plate of connective tissues capable of overlapping posteriorly with the muscles of the pelvic floor.1
Intraoperative bleeding from the exposed bladder muscularis usually is controlled by plication sutures. The bladder pillars contain branches of the inferior vesical arteries, which may require separate ligation. Occasionally profuse venous bleeding arises laterally, from just beyond the visible operative field. If figure-of-eight sutures through the area of venous bleeding are not sufficient, the surgeon may opt to rely on the tamponading by intra-abdominal pressure that occurs normally at the conclusion of the procedure. The advantage of packing the vagina should be weighed against the disadvantage of concealed bleeding. Postoperative bleeding is often due to small active bleeders at the cut edge of the vaginal wall, or from retracted connective tissues just under the mucosal edge; interrupted sutures through the full thickness of the vaginal wall provide necessary control. Occasionally patients are required to return to the operating room for evacuation of a hematoma or ligation of a specific bleeder.
The most common complication after anterior repair is inability to void after the Foley catheter is removed. Generally we remove the catheter on the third or fourth postoperative day. “Kelly-type” plications, which cross the midline underneath the urethra, often delay postoperative voiding. Additional factors include disturbance of bladder innervation, postoperative edema, and spasm of the levator muscles from a concomitantly performed posterior repair. Just before the Foley catheter is removed, a specimen is taken for urine culture. After the catheter is removed early in the morning of the third postoperative day, the patient is instructed to collect voided urine samples using the special receptacles that fit underneath the toilet bowl seat. It is more helpful and less traumatic to measure the amount of voided urine than to catheterize the patient for residual urine. If a woman is able to void more than 100 mL at one sitting, she is not likely to be retaining large volumes within the bladder. Daily retention of 100–200 mL is not unusual during the first postoperative week and may be safely accepted, providing there is no increase in discomfort or palpatory evidence of a distended bladder. If patients are able to void spontaneously for 24 hours without catheterization, their improvement will be progressive, and voiding may be anticipated to continue. If a catheter must be replaced because of a patient's inability to void, coupled with a large volume of retained urine, it should remain in place for at least 5–7 days. Patients generally fare better at home. They should be shown how to remove their own catheter, although a facility should be available should the catheter need to be reinserted. Patients should be reassured that eventually everyone voids.
Some sources have recommended that a suprapubic catheter be placed before the anterior colporrhaphy is performed. The suprapubic tube can be clamped on the first postoperative day, and the patient then should be given the opportunity to void spontaneously. If the patient is able to void after the tube has been clamped for 24 hours, it can be removed with confidence that voiding will continue. If there is no obstruction to the flow of urine from below, the suprapubic opening will close within 1–2 days.
Urinary urge incontinence and detrusor dysfunction may be present for several months after surgery; these are related to the irritation of the bladder muscle by the slow absorption of suture material. Infrequently, incontinence appears as a new symptom after surgery. This type of anatomic defect most often is related to straightening of the urethrovesical angle with consequent posterior rotation of the urethra. Some form of suprapubic urethral suspension usually is required to correct this defect.
ADDENDUM
Anterior Colporrhaphy
The originally described procedure for anterior colporrhaphy remains currently in use, with modification.
Vaginal Colporrhaphy
More recently appreciated anatomic features include designation of the three different levels of vaginal paracolpia. Level 1 consists of the connective tissues at the vaginal apex which adjoin and merge with the sacrouterine ligaments and contribute to support of the vaginal vault. Relaxation of the second, or mid-portion of the paracolpia corresponding to descent of the bladder and vagina, or cystocele. The most distal, anterior portion of the paracolpia, level 3, help to support the urethra and play a role in urinary incontinence.
Vault Mobility
More specifically, with regard to the repair of the anterior vaginal wall, it is possible to evaluate vault mobility more accurately following removal of the uterus. It is our practice, following removal of the uterus, to make a judgment regarding the necessity for specific attachment of the vault to an appropriate structure. We believe an abdominal approach at this stage, by whatever technique, (open, laparoscopic or even robotic) is counter-intuitive. As vault prolapse is often associated with stretching and elongation of the sacrouterine ligaments, reattachment of the vault to these ligaments provides less than consistently satisfactory results. Instead, sutures are now generally placed, at this stage of the operation, through the sacrospinous ligaments (SSL), and then laid aside until completion of the anterior colporrhaphy.
If the vagina is found to be too short to attach comfortably to the SSL, it may be lengthened by appropriating a portion of the vault from the posterior wall, which is usually sufficiently redundant. The sequencing of these operative steps is critical to the success of the operation. If the vagina is not left sufficiently relaxed after its posterior attachment, tension in a posterior direction will affect the integrity of whatever steps are taken under the urethra, i.e. a mesh sling, Kelly sutures, or suprapubic suspensions.
There is a range of anatomic variation; the basic anatomic defect concerns a relaxation of the pelvic floor muscles, resulting in elongation of the ligaments that hold the vaginal vault in place posteriorly. This now requires a more comprehensive evaluation and treatment of anterior compartment defects than was formerly the case.
Recurrences of a Cystocele
Recurrences of a cystocele may still be treated in the standard fashion, but the quality of the native connective tissues must be evaluated and if found deficient, a synthetic mesh plate may be introduced into the potential space between the bladder and vagina, with quadrilateral attachment to the bony or ligamentous portion of the pelvic sidewall. In many instances today the insertion of a mesh plate in this area has become the norm for a primary repair. The findings of mesh erosion and infection , reported to occur in between 10 and 15 % of cases, makes this unjustifiable. Substituting a mesh plate for native tissues may be helpful in cases of massive prolapse in very obese women, where combined weight and force of intra-abdominal pressure cannot hope to be able to control the tendency for recurrent descent. Substitution of mesh for native tissue repair may also be recommended when at least two prior procedures have failed.
REFERENCES
Porges RF: Abnormalities of pelvic support. In Droegmuller W, Sciarra JJ (eds): Gynecology and Obstetrics, Vol 1, Chap 61, addendum, pp 13–15. Philadelphia, JB Lippincott, 1993 |
|
Porges RF, Smilen SW: Long-term analysis of the surgical management of pelvic support defects. Am J Obstet Gynecol 171: 1518, 1994 |